Sometimes, no matter how faithful the steed is, it is time to put it out to pasture. I am starting to find those words ever more meaningful and a tad ironic while in the twilight of my own career. But in this case, there is solace in the fact that the pasture holds rest and hopefully the farmers have been minding the thistles. Applying this theory to some of our most valued equipment, the new year finds us with a new long range fish hauling unit. This new steed has a lot of extras that should not only keep the fish healthier and in better shape, but also be safer and more efficient transport for the fish haulers. The new trailer unit features heavy duty rims and grip strut walkways to increase traction for loading the tanks. Its tank also has a lower profile on the trailer bed, which should allow for lower fuel consumption and less tire wear. The trailer has a new (to Genoa) Ram Air Ventilation system that should alleviate carbon dioxide buildup in some of the long distance hauls that we have currently been performing. It also will have better real time monitoring systems when completed which will allow in route water quality monitoring and correction to reduce physiological stress on the cargo. This new addition to the stable should serve its purpose well as the station’s next generation long hauling distribution unit. By: Doug Aloisi

A dual axle trailer with 1200 gallon tank with 3 separate compartments. Photo: Doug Aloisi/USFWS.
USFWS Pathways and University of Wisconsin La Crosse graduate student, Jadon Motquin will continue his graduate research in 2022 entitled: Evaluation of Genetic Selectivity, Growth and Survival of Lake Sturgeon Fed Commercial and Traditional Diets. This research was made possible with a competitive USFWS research grant and involves collaboration of USFWS researchers from legacy regions 3,4,5 and 6.
Project Goals: 1) To improve aquaculture management practices by optimizing genetic contribution of stocked progeny. 2) Determine if family groups of lake sturgeon habituate to commercially prepared diets at the same rates. 3) Evaluate growth and survival of lake sturgeon fed commercial feed compared to traditional live diets.
(Left) Jadon Motquin USFWS pathways employee collects milt in a syringe for fertilizing lake sturgeon eggs. (Right) Recently fertilized eggs rinsed will hatchery well water and packaged for delivery to GNFH. Photo: USFWS.
This study aims to identify any possible consequences of hatchery feeding practices and identify any possible genetic selectivity that diet may play in cultured lake sturgeon, which could hinder success of lake sturgeon restoration programs. Possible added benefits for the study may potentially identify if commercial diets are an acceptable alternative for lake sturgeon that may improve fish health and reduce fish feed cost at NFHs. The results of this study will improve management practices for lake sturgeon restoration and recovery, potentially accelerating the delisting of this species by continued stocking of lake sturgeon that will have a greater fitness for survival after stocking and be the most genetically fit progeny to restore populations through reproduction and recruitment.
Results from this project will be incorporated into a final manuscript that will be submitted to North American Journal of Aquaculture for publication and made accessible to the public. Reports of results will be shared with stakeholders and information presented at national conferences and regional aquaculture meetings. All data will be incorporated into GNFH’s standard operating procedures for best management practices for lake sturgeon restoration.
By: Orey Eckes

PVC cages with mesh end caps housing Hine’s Emerald Dragonfly larvae. These cages are arranged in flow-through culture tanks in the Dragonfly Trailer at GNFH. Photo: Beth Glidewell/USFWS.

Larvae in culture cups floating in tanks, ready to be packed up for transport.
After spending the growing season in culture tanks in the dragonfly trailer, Hine’s Emerald Dragonfly larvae were checked, photographed for measurement, and packed up for transport during the first week of November. 267 larvae were first placed into pvc tube, mesh screen capped cages in early June. Culture tanks in the trailer allow a fresh supply of pond water and ample zooplankton prey for the larvae, while also creating a semi-controlled culture space where dissolved oxygen, water flow rate and temperature can be monitored and controlled all summer. A mid-season check showed an 81% survival rate for the first stage (cages with 400-500 micron mesh) and an 89% survivorship for second stage cages (the larvae had grown enough that 1mm mesh was sufficient)
The 193 surviving larvae were placed in 100 ml specimen cups for transport. Colleagues from the University of South Dakota (USD) and the Forest Preserve District of DuPage County, IL—partner institutions for the Hine’s Emerald project—came to GNFH and securely packed up the specimen cups for transport back to their institutions for over-winter storage. Water temperatures had dropped to near 40 °F, so larvae had slowed down metabolically and had sufficient oxygen in these cups for transport and will be checked weekly or bi-weekly all winter. Hine’s Emerald larvae typically require several growing seasons to reach a sufficient size that they’ll emerge as adults. These larvae will be cultured for another growing season and their size will be monitored so they can be placed in culture cages that allow both aquatic larval and aerial/perching adult stages well before emergence occurs (the non-aquatic adults very much do not want to be in a mesh cage under water!).
As we said goodbye to one cohort of dragonflies, the next round was arriving. Researchers at USD brought next year’s round of eggs to distribute between GFNH and the Forest Preserve District (with eggs also over-wintering in the USD labs, we’ve got our eggs in 3 baskets…). These eggs will be kept at cold and stable temperatures (3-4°C) until early April, when we’ll begin to warm them and start next year’s hatch.
By: Beth Glidewell
Thank you to everyone that braved the snow to attend the Winter Family Fun Day at the Great River
Road Interpretive Center! We had a great turn out. Attendees got the opportunity to learn about
mussels, ducks, turtles, fish and the Genoa National Fish Hatchery. Also, a huge thank you to all the
presenters and volunteers for making this event happen! A special thanks to the Friends of the Upper
Mississippi, Friends of the Refuge-Mississippi River Pools 7 & 8 and the Upper Mississippi River
National Wildlife & Fish Refuge La Crosse District for providing volunteers and materials for the event!
Attendees were able to take home posters, a gift bag and a fish ornament that they could decorate.
By: Erica Rasmussen
Three volunteers from the Friends of the Upper Mississippi in our gift shop, USFWS staff sharing information on mussels. Photo: Erica Rasmussen/USFWS.


Propagating mussels is difficult for lots of reasons, but their dependence on a live host fish always creates a challenge. Fish survival at the hatchery is usually strong, but the more you handle them, the more prone they are to disease since you damage the protective mucous coat or even dislodge scales and infesting fish with freshwater mussel glochidia involves a lot of handling. Propagation becomes even more arduous when the glochidia transform over months, rather than weeks since you have to keep your fish healthy over long, cold months when they don’t eat much and they’d rather be buried in the mud in a deep river hole. Successfully producing juvenile Winged Mapleleaf, a federally endangered mussel, depends on this over-wintering, as does the Washboard, North America’s largest native freshwater mussel.
Less successful seasons see the loss of these host fish due to disease, and therefore the loss of Winged Mapleleaf larvae, but treatments are required to manage illness, while not harming the developing glochidia. One of our graduate pathways interns has taken this topic on as her graduate thesis work. She is treating Washboard-infested Channel Catfish weekly with typical fish disease treatments to determine whether they decrease the number of quality of juveniles dropping off the fish in the spring. Many treatments have been tested in bass infested with Plain Pocketbook to confirm that they don’t impact the new juveniles, but none have been tested for multiple uses, or over multiple months. Being able to manage disease in Winged Mapleleaf-infested Channel Catfish will make a difference in the success of the recovery program for this species and others across the country.
By: Megan Bradley

Lake Trout eyed eggs. Photo: Erica Rasmussen/USFWS.
As we shared with you in our September edition of Genoa News and Notes, we received Lake Trout eggs from Cayuga Lake in New York that were spawned between October 5-7. The eggs have been incubating since then at 46° F using a chiller unit to cool our well water down from 51° F. Eggs from each set of parents were kept separate from each other. Eggs began hatching on 12/7 and continued until 12/13. Since this is a future broodstock lot, we wanted to maximize the genetic makeup of our lot of fish to the greatest extent possible. We were able to get contributions to the lot from 79 sets of parents for a total of about 1700 fish. These fish will grow up on station until 2023, when they will be sent to their new home at Iron River National Fish Hatchery. There, they will be crossed with other lots of Lake Trout to create a genetically diverse line of production fish that will be stocked to the Great Lakes.
By: Nick Bloomfield

Chiller unit with about 16 trays that are holding about 9 cups filled with eggs. Photo: Erica Rasmussen/USFWS.

Lake Trout with yolk sac visible. Photo: Erica Rasmussen/USFWS.
Volunteers play a vital role in supporting the U.S. Fish and Wildlife Service’s mission: working with others, to conserve, protect and enhance fish, wildlife, and plants and their habitats for the continuing benefit of the American people. The Genoa National Fish Hatchery relies on volunteers to manage the bookstore in the Great River Road Interpretive Center and provide information to the general public about the facility, assist with lake sturgeon and brook trout coded wire tagging, pond harvesting in the spring and fall, mussel cage repairs and tagging of freshwater mussels. Volunteers donate their precious time in helping us at the hatchery complete our mission and for that we are extremely appreciative. Even amid a pandemic, volunteers stepped up to the plate to lend a helping hand while following federal guidelines on mask mandates and social distancing. A combination on 62 volunteers tagged over 60 thousand lake sturgeon and brook trout and donated 229 hours of their time, 12 volunteers harvested fish and mussel ponds and tagged mussels for 25 hours, and 6 volunteers teamed up to staff our visitor center bookstore and volunteered 275 hours while hosting over 1500 visitors from July-October. In just four months of the year, hatchery volunteers donated 529 hours ~ equivalent to 66 eight-hour workdays!!! From the Genoa Staff: “We appreciate all of you for donating your time to make the USFWS mission successful and for supporting our great aquatic resources.” By: Orey Eckes

Volunteers tagging Brook Trout and Lake Sturgeon. Photo: Erica Rasmussen/USFWS.
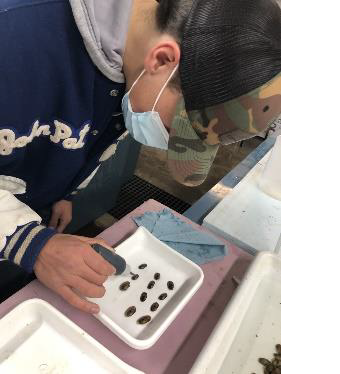
Volunteer tagging Fatmucket mussel. Photo: Erica Rasmussen/USFWS.
2021 was an average year for Mississippi River flows, but after the past few years of high-water flows seemed low. This was especially true in the slough at Blackhawk Park where our Mobile Aquatic Rearing System trailer spends its summer. There were several periods where flows were so low that there wasn’t much, or any, fresh water flowing into the slough from the river and water quality declined several times. As mussels get larger, they’re more resilient to stress because they can stay closed longer, and we saw evidence of this in the Mobile Aquatic Rearing System this year, with greater than 90% survival for our two-year-old and older animals. Survival is always much lower for newly transformed juveniles, and some species had no juveniles survive this summer. Fortunately, we successfully produced many Higgins Eye this year and despite poor survival overall, we collected more than 10,000 juveniles from the trailer in October. These will hopefully continue to grow and survive to be stocked into the Chippewa to continue our reintroduction efforts there.
By: Megan Bradley

Nine, 2-5 millimeter Higgins Eye mussels crawl around in a petri dish during recovery from the Mobile Aquatic Rearing System (MARS trailer). These animals are still tiny and therefore translucent enough that you can see their dark guts while they crawl around seeking different habitats (flow, food and stable sediment). Photo: Megan Bradley/USFWS.

A hatchery tour can be a great destination to explore and learn new things. This was recently the case for the Schiesher sisters. The four sisters live in different locations throughout the United States and every year they get together to explore a new area. This year they elected to explore the driftless region of Western Wisconsin. One of their stops was at the Genoa National Fish Hatchery. They took a tour of the Great River Road Interpretive Center, stopped at the wonderful gift shop, hiked the nature trail and around the ponds and of course, stopped to feed our brook trout from the floating dock! It is so great to see the excitement on everyone’s faces as the fish jump, scrambled and fought to get some food. They walked away with gifts in hand and knowledge and memories to share with others. They wrote very interesting A++ in the address book. Email or call to set up your tour today! By: Erica Rasmussen
(
